The emission spectrum of a light source is the relative light intensity emitted at each particular wavelength. Often times this is plotted in a two dimensional graph with wavelength on the x-axis and light intensity on the y-axis. For visible light sources, the wavelength is often expressed in nanometers (nm) since the visible range of light goes from about 380 nm to about 750 nm. It is sometimes useful to normalize the intensity scale such that the highest value of the y-axis is equal to 1. This is done by dividing all the intensities by the maximum intensity. This normalization process eases the comparison of different intensity light sources. Here are the emission spectrums of 4 different color LEDs.
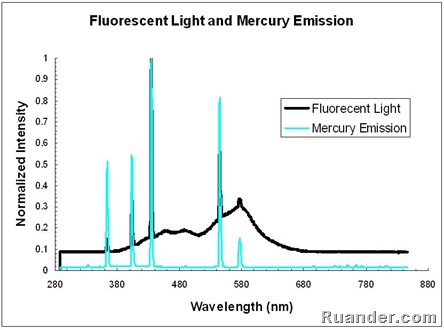
A fluorescent light source emits light from a gaseous mercury source as well as from a fluorescent phosphor coating which absorbs the UV emissions from the mercury and emits in the visible range. This process can be seen in the following graph that shows both the emission from a fluorescent light and the emission form a pure mercury source. Note that the peaks of the Mercury emission are also seen in the Fluorescent light. However, the fluorescent light also shows emission in between peaks due to the phosphor coating emission.
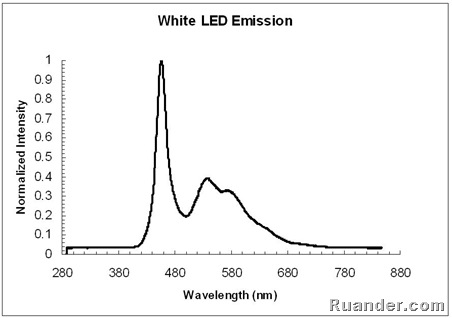
Here is the emission spectrum of a white LED. Note that the shape of the spectrum is very different from the other 4 LEDs shown above.
White LEDs work much similar to a Fluorescent light. They use a blue LED source and a phosphor coating that absorbs a fraction of the blue LED emission and emits in other visible wavelengths such as green and yellow. This can be seen by superimposing the emission spectrum of a white LED, a blue LED and the Fluorescent light spectrum. Note that the White LED spectrum looks similar to the blue LED spectrum plus the Fluorescent light spectrum without the Mercury peaks.
Here are the spectrum emission of 3 different color Lasers: a red and a green laser pointer and a pink Helium-Neon laser. Note that the width of the spectrum of a laser source is much narrower than all the other sources. In fact, in some instances the width of the spectrum is even smaller than shown here; however, the accuracy of the spectrometer used was not enough to capture the very narrow spectral width. This characteristic of laser sources is called monochromatic light, which means that laser light is ideally composed of only 1 wavelength.




Woow - thanks Ruander. Is there any way of getting hold of the raw data?
ReplyDeleterocket science for me... understand almost nothing
ReplyDelete